|
Frequently Asked Questions (FAQ) About Batteries
BATTERIES NEVER DIE....THEY ARE MURDERED!
- How are batteries rated and what do the ratings mean in battery selection?
- How does the Cold Cranking Amperage rating help me select a battery?
- What does the Reserve Capacity rating mean and how does it apply to deep cycle batteries?
- What is battery cycle life?
- What is the difference between deep cycle batteries and starting batteries?
- What is the difference between series battery connections and parallel battery connections?
- Does overcharging damage batteries?
- Does overdischarging damage batteries?
- How can I evaluate the health and charge state of a battery?
FAQ List
1) BATTERY RATINGS
How are batteries rated and what do the ratings mean in battery selection?
ANSWER:
The most common battery rating is the AMP-HOUR RATING. This is a unit of
measurement for battery capacity, obtained by multiplying a current flow in
amperes by the time in hours of discharge. (Example: A battery which
delivers 5 amperes for 20 hours delivers 5 amperes times 20 hours, or 100
ampere-hours.)
Manufacturers use different discharge periods to yield an different Amp-Hr.
Rating for the same capacity batteries, therefore, the Amp-Hr. Rating has
little significance unless qualified by the number of hours the battery is
discharged. For this reason Amp-Hour Ratings are only a general method of
evaluating a battery's capacity for selection purposes. The quality of
internal components and technical construction within the battery will
generate different desired characteristics without effecting its Amp-Hour
Rating. For instance, there are 150 Amp-Hour batteries that will not
support an electrical load overnight and if called upon to do so
repetitively, will fail early in their life. Conversely, there are 150
Amp-Hour batteries that will operate an electrical load for several days
before needing recharging and will do so for years. The following ratings
must be examined in order to evaluate and select the proper battery for a
specific application: COLD CRANKING AMPERAGE and RESERVE CAPACITY are
ratings used by the industry to simplify battery selection.
FAQ List
2) COLD CRANKING AMPERAGE:
How does the Cold Cranking Amperage rating help me select a battery?
ANSWER:
(CCA) is the maximum amperes that can be continuously removed from a battery for 30 seconds at 0°F before its
voltage drops to unusable levels. A 550 CCA battery can supply 550 amperes
for 30 seconds at 0°F. This rating is only useful in the selection of
engine starting batteries.
NOTE: Do not confuse Cold Cranking Amperage (CCA) with Marine Cranking
Amperage (MCA) or Cranking Amperage (CA). MCA and CA is a higher battery
rating measured at warmer temperatures.
FAQ List
3) RESERVE CAPACITY
What does the Reserve Capacity rating mean and how does it apply to deep cycle batteries?
ANSWER:
Reserve capacity is the number of minutes a battery can maintain a useful
voltage under a 25 ampere discharge. The higher the minute rating, the
greater the battery's ability to run lights, pumps, inverters, and
electronics for a longer period before recharging is necessary. The 25
Amp. Reserve Capacity Rating is more realistic than Amp-Hour or CCA as a
measurement of capacity for deep cycle service. Batteries promoted on
their high Cold Cranking Ratings are easy and inexpensive to build. The
market is flooded with them, however their Reserve Capacity, Cycle Life
(the number of discharges and charges the battery can deliver) and Service
life are poor. Reserve Capacity is difficult and costly to engineer into a
battery and requires higher quality cell materials.
For instance, Rolls, Surrette and Lifeline use thicker lead grids (the
plate's skeletal structure) to support additional positive plate oxides
which are compressed into a denser form in order to add battery reactive
material for greater Reserve Capacity and Cycling Performance. In
addition, these plates are separated by indestructible separators. These
mats hold the active oxides tightly in place during the cubical plate
expansion which occurs during deep discharging, instead of allowing the
oxides to shed off and precipitate to the bottom of the battery.
Construction materials such as those raise the Reserve Capacity of a
battery and increase the battery's Cycle Life.
FAQ List
4) CYCLE LIFE
What is battery cycle life?
ANSWER:
One cycle of a battery is a discharge from full charge to full discharge
and a return to full charge again. The total number of cycles a battery
can perform before failure is called its Cycle Life. Most battery
manufacturers will not discus the Cycle Life of their product. Many
advertised Deep Cycle batteries have not been tested, or, which is the case
with cranking batteries, were never designed for long Cycle Life .
FAQ List
5) DEEP CYCLE BATTERIES
What is the difference between deep cycle batteries and starting batteries?
ANSWER:
Unfortunately, the term Deep Cycle has been overused by the battery
industry as a sales tool to imply a heavy duty product. This has led to
confusion and difficulty in battery selection. One must understand that
any battery may be termed deep cycle as all batteries may be fully
discharged and charged. However, a true deep cycle battery, such as Rolls
or Lifeline, is capable of thousands of these hard cycles during its life
without losing its capacity. Comparatively, many advertised deep cycle
batteries composed of thin plates, excessively porous separators, and low
density plate oxides will suffer permanent capacity loss after a few dozen
cycles and will shortly sulfate or shed plate material and fail. Batteries
without substantial materials designed for true deep-cycling will lose more
than half of their capacity after only a few cycles. A 200 Amp-hour
battery will shortly become a 100 Amp-hour battery for the remainder of its
shortened service life. What initially may seem to be an inexpensive
battery to purchase, now costs twice as much per Amp-hour.
True Deep cycle batteries will perform well as cranking batteries, however,
cranking batteries will not survive deep cycle use.
Deep cycle batteries can be used in any application and exhibit a long
service life, while cranking batteries are limited to starting applications
only. Cranking batteries exhibit poor service life in cycling
applications.
FAQ List
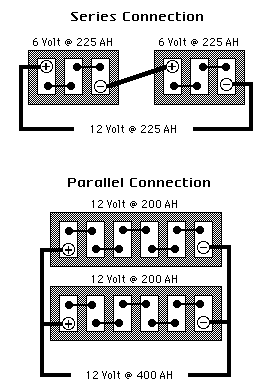
6) INCREASING CAPACITY THROUGH SERIES AND PARALLEL CONNECTIONS
What is the difference between series battery connections and parallel battery connections and how do they increase battery capacity and voltage?
ANSWER:
In the SERIES CONNECTION, batteries of like voltage and Amp-Hour capacity
are connected to increase the Voltage of the bank. The positive terminal
of the first battery is connected to the negative terminal of the second
battery and so on, until the desired voltage is reached. The final Voltage
is the sum of all battery voltages added together while the final Amp-Hours
remains unchanged. The bank's Voltage increases while its Amp-Hours,
Cranking Performance and Reserve Capacity remain unchanged.
In the PARALLEL CONNECTION, batteries of like voltages and capacities are
connected to increase the capacity of the bank. The positive terminals of
all batteries are connected together, or to a common conductor, and all
negative terminals are connected in the same manner. The final voltage
remains unchanged while the capacity of the bank is the sum of the
capacities of the individual batteries of this connection. Amp-Hours
Cranking Performance and Reserve Capacity increases while Voltage does not.
FAQ List
7) BATTERY MAINTENANCE
Does overcharging damage batteries?
ANSWER:
OVERCHARGING is the most destructive element in battery service. Usually
the boater is not aware that this is occurring as he believes his
alternator or battery charger is "automatic." Unfortunately, these
automatic circuits are sensitive to voltage surges, heat, direct lightening
strikes and indirect lightening electromagnetic influences and could fail
or shift their calibration. When they fail, overcharging begins to effect
the batteries. During overcharging, excessive current causes the oxides on
the plates of the battery to "shed" and precipitate to the bottom of the
cell and also heat the battery, thus removing water from the electrolyte.
Once removed, this material (which represents capacity) is no longer active
in the battery. In addition, the loss of water from the electrolyte may
expose portions of the plates and cause the exposed areas to oxidize and
become inactive, thus reducing additional capacity.
Sealed batteries are not immune from the same internal results when
overcharged. In fact, sealed recombination absorption and gel batteries
are particularly sensitive to overcharging. Once moisture is removed from
the battery, it cannot be replaced. Portions of the battery damaged due to
overcharging are irretrievable. However, if detected early, corrective
adjustments to the charging device will save the undamaged portion of the
battery. Initial signs of overcharging are excessive usage of water in the
battery, continuously warm batteries, or higher than normal battery
voltages while under the influence of the charger. If overcharging is
suspected, correct immediately.
FAQ List
8) OVERDISCHARGING
Does overdischarging damage batteries?
ANSWER:
OVERDISCHARGING is a problem which originates from insufficient battery
capacity causing the batteries to be overworked. Discharges deeper than
50% (in reality well below 12.0 Volts or 1.200 Specific Gravity)
significantly shorten the Cycle Life of a battery without increasing the
usable depth of cycle. Infrequent or inadequate complete recharging can
also cause overdischarging symptoms called SULFATION. Despite that
charging equipment is regulating back properly, overdischarging symptoms
are displayed as loss of battery capacity and lower than normal specific
gravity. Sulfation occurs when sulfur from the electrolyte combines with
the lead on the plates and forms lead-sulfate. Once this condition becomes
chronic, marine battery chargers will not remove the hardened sulfate.
Sulfation can usually be removed by a proper desulfation or equalization
charge with external manual battery chargers. To accomplish this task, the
flooded plate batteries must be charged at 6 to 10 amps. at 2.4 to 2.5
volts per cell until all cells are gassing freely and their specific
gravity returns to their full charge concentration. Sealed AGM batteries
should be brought to 2.35 volts per cell and then discharged to 1.75 volts
per cell and their this process must be repeated until the capacity returns
to the battery. Gel batteries may not recover. In most cases, the battery
may be returned to complete its service life.
CHARGING
Alternators and float battery chargers including regulated photo voltaic
chargers have automatic controls which taper the charge rate as the
batteries come up in charge. It should be noted that a decrease to a few
amperes while charging does not mean that the batteries have been fully
charged. Battery chargers are of three types. There is the manual type,
the trickle type, and the automatic switcher type.
FAQ List
9) BATTERY ELECTROCHEMISTRY EVALUATION
How can I evaluate the health and charge state of a battery?
ANSWER:
Routine battery examinations divulge irregularities in the charging system
as well as in the batteries. The principle method is to examine the
electrochemistry of the battery through hydrometric electrolyte inspection.
As previously discussed, this important examination cannot be accomplished
with sealed absorption or gel batteries. Voltage readings alone require
experience to interpret. Hydrometric readings will uncover early warnings
of overcharging or overdischarging before batteries are damaged.
The state-of-charge and reliability of a lead acid battery can best be
determined by the specific gravity of the electrolyte measured directly
with a common bulb-type hydrometer with a glass float. We do not recommend
the ball float type hydrometer. Specific gravity is a unit of measurement
for determining the sulfuric acid content of the electrolyte. The
recommended fully charged specific gravity of marine batteries is 1.255 to 1.265 taken at 80°F. More than .025 spread in readings between fully
charged cells indicates that the battery may need an equalization charge.
If this condition persists, the cell is failing and the battery should be
replaced. Since water has a value of 1.000, electrolyte with a specific
gravity of 1.260 means it is 1.260 times heavier than pure water while pure
concentrated sulfuric acid has a specific gravity of 1.835.
The following table illustrates typical specific gravity values for a cell
in various stages of charge:
100% Charged.......1.255 - 1.260 Sp. Gr.
75% Charged.......1.220 - 1.225 Sp. Gr.
50% Charged.......1.185 - 1.190 Sp. Gr.
25% Charged.......1.150 - 1.155 Sp. Gr.
0% Charged.......1.115 - 1.120 Sp. Gr.
Temperature compensation of hydrometric readings is usually unnecessary
unless the battery is extremely hot or cold, however, after hard charging
or discharging, you may want to add or subtract points of Specific Gravity
based on the table.
(PICTURE OF THERMOMETER)
Do not apply hydrometer color coding to readings taken from deep cycle
batteries. These red-white-green markings are for "hot" automotive battery
types. Also, hydrometer readings taken immediately after water is added to
a cell is inaccurate. The water must be thoroughly mixed with the
underlying electrolyte by charging, before hydrometer readings are
reliable. In addition, do not assume a deep cycle battery will not take a
charge because you have been charging it for a while and the float will not
rise. If the battery has been fully discharged or partially sulfated it
will require considerable charging or equalization before recovering.
As electrolyte levels are reduced in the battery, it is important to add
water to each cell. Note that only the water portion of the electrolyte
evaporates, therefore, it is not necessary to add acid to a battery during
maintenance. In fact, the addition of acid to an active battery will
reduce its capacity and shorten its remaining life. Water should be added
to cells after charging the battery. This will eliminate spillage due to
expansion of electrolyte upon charging. Generally speaking, any water that
is safe to drink is safe to use in a battery. Do not use water of a known
high mineral content or stored in metallic containers. It is the metal
impurities in the water that lower the performance of the battery.
Distilled water guarantees purity.
FAQ List
NOTE:
Frequently Asked Questions (FAQ) section designed and copyrighted © 1996 by
DC Battery Specialists. All rights reserved. No part may be
reproduced for publication on the Internet or other publication
without the expressed consent of DC Battery Specialists.
|

